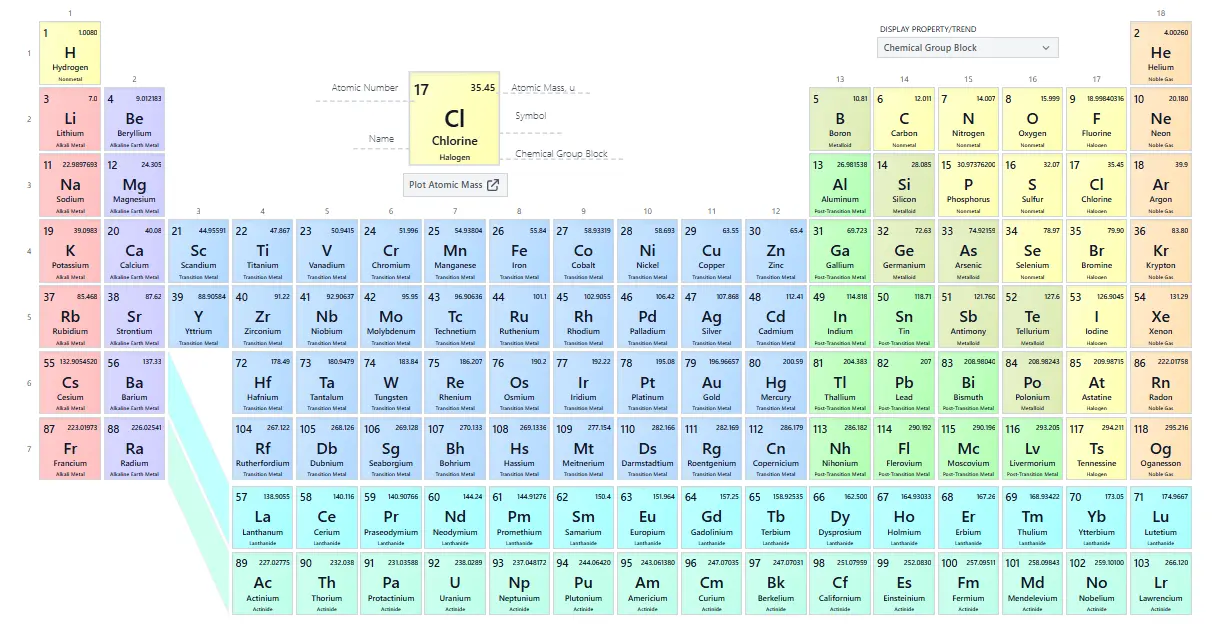
Periodic Table of Elements
The Periodic Table of Elements is one of the most iconic and essential tools in science. It organizes all known chemical elements in a systematic way that reveals patterns in their properties and behaviors. Whether you’re a student, a science enthusiast, or just curious about the building blocks of matter, understanding the periodic table unlocks a whole new world of chemistry.
What Is the Periodic Table?
The periodic table is a tabular arrangement of chemical elements, organized by their atomic number, electron configurations, and recurring chemical properties. Elements are listed in order of increasing atomic number the number of protons in an atom’s nucleus and grouped into rows (periods) and columns (groups or families) that share similar characteristics.
A Brief History of the Periodic Table
- Early Attempts: Before the periodic table, scientists struggled to organize the elements discovered up to that point. In the early 1800s, elements were arranged by atomic weight, but no clear pattern emerged.
- Dmitri Mendeleev: In 1869, Russian chemist Dmitri Mendeleev created the first widely recognized periodic table. He arranged elements by increasing atomic weight but also grouped them based on similar chemical properties. Remarkably, Mendeleev predicted the existence and properties of elements that hadn’t been discovered yet, leaving gaps in his table as placeholders.
- Modern Periodic Table: With the discovery of the atomic number and understanding of atomic structure in the early 20th century, the table was rearranged by atomic number rather than atomic weight. This correction resolved inconsistencies and formed the basis of the modern periodic table we use today.
Structure of the Periodic Table
Periods
- The table has 7 horizontal rows called periods.
- Each period corresponds to the filling of a different electron shell.
- As you move from left to right across a period, elements gain one proton and one electron at a time.
Groups or Families
- The 18 vertical columns are called groups or families.
- Elements in the same group share similar chemical and physical properties because they have the same number of electrons in their outer shell (valence electrons).
- Examples include the alkali metals in Group 1, alkaline earth metals in Group 2, halogens in Group 17, and the noble gases in Group 18.
Blocks
The periodic table is also divided into blocks based on the electron configuration:
- s-block: Groups 1 and 2, plus helium.
- p-block: Groups 13 to 18.
- d-block: Transition metals in groups 3 to 12.
- f-block: Lanthanides and actinides, placed below the main table.
Why Is the Periodic Table Important?
- Predicting Properties: The periodic table allows scientists to predict the chemical behavior of elements, even those not yet discovered.
- Understanding Reactions: It helps chemists understand how elements will react with each other.
- Classification Tool: It categorizes elements into metals, nonmetals, and metalloids, which aids in understanding their uses and physical characteristics.
- Foundation for Chemistry: From high school classrooms to cutting-edge research labs, the periodic table is the backbone of chemistry education and innovation.
Fun Facts About the Periodic Table
- The periodic table currently includes 118 confirmed elements, with the latest additions being elements 113 (Nihonium), 114 (Flerovium), 115 (Moscovium), 116 (Livermorium), 117 (Tennessine), and 118 (Oganesson).
- The only metal that is liquid at room temperature is mercury (Hg).
- Hydrogen (H), the simplest element, doesn’t quite fit neatly into any group.
- Some elements are named after places (Californium, Europium), scientists (Einsteinium, Curium), or mythological figures (Thorium).
How to Read the Periodic Table
Each element box on the periodic table typically shows:
- Atomic Number: Number of protons in the nucleus.
- Element Symbol: One or two-letter abbreviation (O for oxygen).
- Element Name: Full name of the element.
- Atomic Mass: Average mass of atoms of that element, considering isotopes.
Conclusion
The periodic table is much more than just a chart; it’s a powerful scientific tool that continues to evolve as we discover new elements and better understand atomic structure. It’s a gateway to exploring the vast diversity of matter that makes up everything in the universe from the air we breathe to the stars in the sky.